Hydroxychloroquine Retinal Toxicity
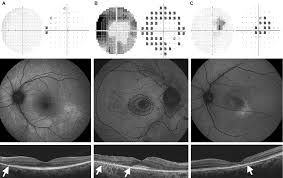
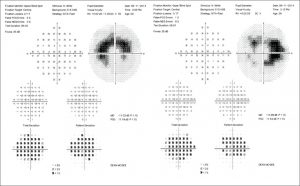
Figure 1. Ocular findings of (A) pericentral pattern, (B) mixed pattern, and (C) parafoveal pattern of hydroxychloroquine retinal toxicity. (A) Top to bottom: Superior field loss is located peripherally in pattern deviation plot (10-2 visual field). FAF image shows a broad range of hyperautofluorescence in inferior retina. SD-OCT image shows loss of photoreceptor layer in the pericentral area (arrow). (B) Top to bottom: 10-2 visual fields (threshold and pattern deviation plot) are showing full ring scotomas. FAF image shows ring-shaped hyperautofluorescence in the parafoveal area (dashed curves) with diffuse hyperautofluorescence in the pericentral area (dashed curves). SD-OCT image shows disruption of the photoreceptor layer with loss of outer nuclear layer in both parafoveal and pericentral retina (arrows). RPE damage is combined in the extrafoveal region. (C) Top to bottom: 10-2 visual fields (threshold and pattern deviation plot) are showing patchy parafoveal scotomas. Hyperautofluorescence is shown at a region just inferior to macula on FAF image. SD-OCT image shows parafoveal disruption of photoreceptor layer with loss of outer nuclear layer (arrow). RPE damage is suspected within the parafoveal retina. FAF: fundus autofluorescence; SD-OCT: spectral domain optical coherence tomography; RPE: retinal pigment epithelium.
Retinal Toxicity Progression
Severe HCQ retinopathy often progresses even after drug cessation
Researchers used multimodal imaging to study retinal and vision changes after hydroxychloroquine (HCQ) cessation in 22 patients with HCQ retinal toxicity.
Their analysis revealed that eyes with HCQ retinopathy experience progressive changes in retinal structure and function after discontinuing treatment, and the magnitude of these changes reflects the severity of retinopathy.
Eyes with subtle, localized retinopathy, however, tend to remain stable and may gain functional improvement. Early detection of HCQ retinopathy is needed to stop retinopathy progression and improve long-term outcomes. Retina, March 2019
Hydroxychloroquine-Induced Retinal Toxicity
This article is from June 2011 and may contain outdated material.
Many systemic medications may cause retinal toxicity. One such commonly used medication for dermatologic and rheumatologic inflammatory conditions is hydroxychloroquine (Plaquenil), a chloroquine derivative. It is used to treat many diseases including malaria, rheumatoid arthritis and systemic lupus erythematosus.
Retinal toxicity from hydroxychloroquine is rare, but even if the medication is discontinued, vision loss may be irreversible and may continue to progress. It is imperative that patients and physicians are aware of and watch for this drug’s ocular side effects. And before treatment is initiated with hydroxychloroquine, a complete ophthalmic examination should be performed to determine any baseline maculopathy.
Ophthalmologists should also follow the most current screening guidelines established by the Academy,1 recently revised in light of new findings.
Symptoms and Signs of Chloroquine Toxicity
Symptoms. Patients in earlier stages of hydroxychloroquine retinal toxicity usually do not experience symptoms, though the rare patient may note a paracentral scotoma that causes trouble with reading as well as diminished color vision. However, most patients usually notice symptoms only after scotomas have become severe. When allowed to advance, hydroxychloroquine retinal toxicity leads to loss of up to three visual functions: acuity, peripheral vision and night vision.
Signs. On examination, a telltale sign of hydroxychloroquine toxicity is a bilateral change in the retinal pigment epithelium of the macula that gives the commonly described appearance of a bull’s-eye. This is a late finding, however, and too late for screening to be useful.
In early toxicity there are no visible signs, but field, OCT and mfERG changes can be detected. If abnormalities are present only unilaterally, investigate other causes besides hydroxychloroquine toxicity (see “Differential Diagnosis of Bull’s-Eye Maculopathy”).
Mechanism of HCQ Toxicity
The mechanism of hydroxychloroquine retinal toxicity has yet to be fully elucidated. Studies have shown that the drug affects the metabolism of retinal cells and also binds to melanin in the RPE, which could explain the persistent toxicity after discontinuation of the medication. However, these findings do not explain the clinical pigmentary changes causing a bull’s-eye maculopathy.
HCQ Medication Dosage
Several factors have been associated with the risk of developing hydroxychloroquine retinopathy. One of the most important appears to be dosage—with debate over whether daily intake vs. cumulative dosage is most significant. Recent studies indicate that cumulative dosage may be a more important consideration than daily dosage.2 However, since higher daily dosage will obviously lead to the toxic cumulative dose more rapidly, daily dosage is still important to consider. Higher daily dosage also leads to higher concentration of the drug in the RPE, which could lead to more aggressive tissue damage. Previous reports indicate that toxicity is rare if dosing is less than 6.5 mg/kg/day.2 To avoid overdosage, especially in obese patients or those of short stature, dose should be based on height, which allows for an estimation of ideal body weight. (The drug clears slowly from the blood, so basing dosage on weight puts obese patients at risk.) The typical daily dosage for most indications is 200 mg to 400 mg per day. Daily dosage is recommended not to exceed 400 mg.
Risk for HCQ Toxicity
Although it is not possible to predict which patients will develop retinal toxicity, high-risk characteristics include the following:
- daily dose greater than 400 mg (or, in people of short stature, a daily dosage over 6.5 mg/kg ideal body weight) or total cumulative dose of more than 1,000 g
- medication use longer than five years
- concomitant renal or liver disease (because the drug is cleared by both routes)
- underlying retinal disease or maculopathy
- age greater than 60 years.
Monitoring Guidelines for Chloroquine Toxicity
Guidelines on screening for retinopathy associated with hydroxychloroquine toxicity were initially published by the Academy in 2002. These guidelines were updated in February of this year, given the emergence of more sensitive diagnostic techniques and the recognition that risk of toxicity from years of hydroxychloroquine use is greater than previously believed.
The updated guidelines state that due to sensitivity, specificity and reliability issues, it is not recommended that Amsler grid testing, colour vision testing, fundus examination and full-field electroretinogram or electrooculogram be used for toxicity screening. Fluorescein angiography may assist in visualizing early subtle changes in the RPE, but it is not considered a screening tool for retinal toxicity.
It is critical to counsel patients about the benefits and limitations of screening, underscoring that it can catch toxicity at early stages and minimize vision loss but cannot necessarily prevent all toxicity and vision loss.
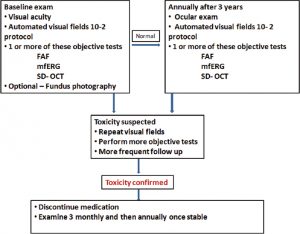
Baseline examination. At the initiation of treatment with hydroxychloroquine, the prescribing physician should refer the patient to an ophthalmologist. During the initial examination, it is recommended that the patient receive:
- 1) a thorough ocular examination documenting any preexisting conditions,
- 2) a Humphrey visual field central 10-2 white-on-white pattern, and
- 3) at least one of the following objective tests, if available:
-
- fundus autofluorescence (FAF)
- multifocal electroretinogram (mfERG) or
- spectral domain OCT (SD-OCT).
In fact, mfERG—a test that is typically available in large clinical centers—objectively evaluates the function and can be used in place of visual fields. It’s also worth considering the use of colour fundus photographs to assist in documenting changes over time, especially if there is preexisting retinal pathology. However, the dilated fundus exam should not be considered a screening tool, as it only picks up relatively late toxic changes.
Ongoing monitoring. Encourage the patient to obtain an annual ophthalmic examination as part of the screening process. Since toxicity is rare within the first five years of treatment, ancillary testing is not necessary unless abnormalities are noted on baseline examination. However, earlier, more frequent screening may be prudent for those at higher risk for toxicity. After five years of treatment, perform annual screenings, including an ocular examination, 10-2 threshold field testing, and one of the objective tests. In practical terms, SD-OCT is most widely available and is very sensitive, so practitioners should look for subtle parafoveal abnormalities.
Toxicity: suspected and confirmed. Whenever you note abnormalities, obtain additional testing. Repeat visual fields promptly if you see central or parafoveal changes, even if these appear to be nonspecific. If these findings are reproducible, follow up with objective testing. If toxicity is suspected, perform more frequent and detailed examinations. Once toxicity is confirmed, the prescribing physician should be notified and hydroxychloroquine discontinued unless it is medically critical and the patient has been informed of the visual risk. Before discontinuation, inform the patient that the drug clears slowly from the body and therefore visual function may continue to slowly deteriorate.
Differential Diagnosis of Bull’s-Eye MaculopathyAge-related macular degeneration Benign concentric annular dystrophy Central areolar choroidal dystrophy Chloroquine/hydroxychloroquine retinal toxicity Chronic macular hole Cone and cone-rod dystrophies Stargardt disease |
Conclusion
Patients and their physicians prescribing hydroxychloroquine need to be keenly aware of retinal toxicity risks and the importance of regular screening, and ophthalmologists who see these patients should keep retinal toxicity in the front of their minds. Adhering to the Academy’s guidelines will help achieve the goal of identifying abnormalities with screenings and examination prior to the patient’s visual complaints.
___________________________
1 Marmor, M. F. et al. Ophthalmology 2011;118:415–422.
2 Mieler, W. F. New Monitoring Guidelines for Hydroxychloroquine. Presented at Retina Subspecialty Day, Oct. 16, 2010, Chicago.